Projects
Research projects
Explore below the ongoing and finalized research projects for Master and PhD students.
Research projects
Project description
Radiotherapy is one of the pillars to treat oncological malignancies. Targeted radionuclide therapy (TRT) is emerging as a promising treatment strategy as well. Unfortunately, resistance to radiation-based therapies remains a clinical challenge. The combination of radiation-based therapies and immune checkpoint inhibitors has synergistic potential and could help to overcome this hurdle.
The effects of radiation on the immune system are of paramount clinical importance. The mechanism between radiation-induced cell damage and immune cells is complex and balances between immune-stimulatory and -inhibitory effects which strongly influence antitumor activity (figure).
Establishing animal models to uncover mechanisms driving radiation resistance in cancer is essential to improve radiopharmaceutical drug development. Evaluating radiation-based therapies in murine tumor models with an intact immune system will help us gain greater understanding of radiation biology, resistance mechanisms, and interaction with immune cells. With this project we are therefore leveraging our expertise in the field.
Project objectives
The objective of this project is to evaluate radiosensitivity in a panel of clinically-relevant murine syngeneic tumor models and the effect of combining radiotherapy and immune checkpoint inhibition on treatment resistance.
- In this project you will first establish a library of 5-8 syngeneic mouse models investigating murine tumor sensitivity to external beam radiation (EBR) in immunocompetent mice.
- Then, 1-2 models (sensitive vs. resistant) will be selected for combination treatment studies of EBR with common immunotherapies, and radiosensitizers.
- Ex vivo methods on tumors, lymph nodes, and blood will be used to evaluate immune activation and DNA damage response, such as flow cytometry, IHC, IDEXX hematology.
- If the project allows it, a combination treatment from part II that has shown synergy will be repeated, and mice will undergo molecular imaging to visualize positive CD8 cytotoxic T cells.
Applied methods
- Culturing of cancer cells
- Subcutaneous inoculation of cancer cells
- Treatment with external beam radiation
- Treatment with immune checkpoint inhibitors and radiosensitizers
- In vivo tumor growth monitoring by caliper measurements
- Ex vivo evaluation of tumor microenvironment e.g. flow cytometry
- If relevant, µPET/CT imaging
Project description
Targeted radiopharmaceutical therapies (TRT) have recently opened new avenues for cancer patients to the extent where radiopharmaceutical drugs have been forecasted to “storm the market”.1
Contrary to conventional radiation therapy which delivers beams of radiation from outside the body to kill tumors inside the body (Figure 1a), TRT delivers specific radiation to tumors and prospective metastasis throughout the body while sparring healthy tissues (Figure 1b).
Most novel TRTs are excreted through the renal system, and in some instances, reabsorbed into the kidney leading to increased radiation exposure and damage to the kidneys (Figure 1c). The treatment may be excellent at eradicating tumors, but the kidney toxicity will be a showstopper for the drug moving to the clinic.
At Minerva Imaging, we want to be the global partner supporting the development of radiopharmaceutical therapies for cancer. To select the most effective and safe drugs, we wish to develop methods to evaluate TRT induced kidney toxicity ultimately guiding and accelerating radiopharmaceutical drug development.

Figure 1 . Dolgin, E. Radioactive drugs emerge from the shadow to storm the market (2018). Nature Biotechnol. 36(12):1125-1127. doi: 10.1038/nbt1218-1125.
Project objectives
The project aims to develop methods to assess nephrotoxicity after TRT in mice and potentially also minipigs. The objective is to elucidate and establish protocols for assessing key parameters of kidney function and injury by leveraging on various state-of-the-art in vivo and ex vivo equipment and assays. Throughout the project period, the student will have a significant role in identifying and determining key parameters to assess and will get hands-on experience with a variety of methods across our interdisciplinary pharmacology departments, including but not limited to:
- Establishing animal models of late-stage radiation (kidney) toxicity: Treatment planning and administration of single and repeated dose regimens of radiopharmaceutical therapy in animals
- Animal monitoring: Body weight monitoring and establishing clinical observations protocols
- Blood sampling, hematology readouts and plasma assays: Longitudinal assessment of influence on blood parameters and biomarkers linked to renal function/injury
- Non-invasive in vivo imaging: Longitudinal assessment of kidney function with diagnostic tracers, developing optimal imaging protocols
- Macroscopic examinations: Necropsy and organ weights to assess extend of tissue damage
- Microscopic examinations: Immunohistochemistry and autoradiography of kidney tissue sections to evaluate severity of damage with AI software
Project description
Targeted radionuclide therapy (TRT) is an emerging cancer treatment that delivers targeted radiation to tumors. As several radioligand-based drugs will soon become standard-of-care treatments, RLT has recently gained significant attention as a key area of research and development within the pharmaceutical industry. With the increasing clinical use of TRT it becomes evident that some patients do not benefit from the therapy, while others develop a resistance to the therapy after an initial response. Hence, there is a growing interest in understanding the mechanisms of resistance to TRT and the need to develop therapies that are able to enhance its therapeutic effect. As the primary mechanisms of action of TRT is DNA damage, the investigation of DNA damage response (DDR) becomes crucial for optimizing treatment strategies. At Minerva Imaging, we provide validated immunohistochemistry staining protocols for key markers of DNA double-strand breakage to our clients (Figure 1), and we wish to expand this selection with other markers of DNA damage.
Establishing models for radioligand therapy is essential to uncover the mechanisms driving treatment resistance in cancer. We therefore want to develop cancer cell line models to investigate molecular and cellular changes that enable the cells to survive and adapt to TRT. Moreover, the cell lines will be ideal models to screen for TRT sensitizing molecular targets in high-throughput assays.

Figure 1. DNA double strand breaks visualization by staining for γH2AX. Left, γH2AX (purple) in cell nuclei (blue) treated with increasing radiation doses (Gy). Right, quantification of the γH2AX signal by Flow cytometry and Immunocytochemistry (ICC).
Project objectives
In this project, we will develop different TRT resistant cancer cell line models and in parallel, develop protocols for analyzing DDR marker using histological evaluation and/or flow cytometry. We will then evaluate the DDR of the sensitive and resistant cell line to understand the mechanism of resistance. Finally, and if time, the cells will be included in a screen of known radiosensitizer together with TRT to find possible therapeutic strategies.
Project description:
Glioblastoma multiform (GBM) is the most common tumor type in the brain and is associated with a very poor prognosis. The main challenges in GBM therapy stem from its location, invasive nature, and resistance to therapies. Consequently, it is of high demand to overcome resistance mechanisms and find new treatments for GBM. To this end it is crucial to use clinically relevant animal models, that mimic the disease and allow for fast and cheap analysis.
We therefore developed at Minerva Imaging a glioblastoma cell line from a patient derived xenograft (PDX) model, that maintained the clinical features of the original tumour including resistance to radiation. Together with Professor Krister Wennerberg’s laboratory at BRIC, we have used this cell line to screen for new treatment agents, as well as compounds that could be used for re-sensitizing the cell line to radiation.
In this project, Monica Brandt evaluated some of the potential treatment agents and radiosensitizers for GBM and identified their mechanism of action. We evaluated the effectiveness of potential new treatments and radiosensitizers on the PDX-derived GBM cell line. The project was a collaboration between Minerva Imaging and Krister Wennerberg’s laboratory at BRIC at Copenhagen University.
Applied methods:
- Collection and preservation of orthotopic tumors from mice
- Ex vivo analysis of tumor tissue
- Cell culturing of cancer cells in 2D and/or 3D
- Viability measurements
- Signaling pathway analysis
The blood brain barrier serves as a strict filter limiting the diffusion of cells, metabolites, and toxins into the brain. The barrier also hinders the delivery of therapies to diseased sites in the brain. This complicates the development of novel therapies targeting e.g. brain cancer and brain metastasis and increases cost and time requirements. Relevant animal models accurately reflecting the complex role of the blood brain barrier are of high importance for the development of Central Nervous System (CNS) targeting therapies.
This industrial PhD project aimed to develop a comprehensive platform, with high translation value, for evaluating the ability of drugs to cross the blood brain barrier, reach their target in the brain, and exert their function.
We generated advanced in vitro and in vivo models recapitulating the complexity of the blood brain barrier in different settings. In vitro models were developed using cells normally constituting the blood brain barrier in patients while in vivo models were developed by employing various methods to establish orthotopic brain cancer in mice. Furthermore, we established robust non-invasive imaging protocols using bioluminescence, magnetic resonance imaging, SPECT/CT, and PET/CT for evaluating drug delivery and accumulation to the brain aiding in accelerating the successful development of novel CNS-targeting drugs.
Immunotherapy is a very successful and rapidly evolving strategy for the treatment of cancer. Adoptive cell transfer is an advanced type of immunotherapy that is gaining increasing interest as it utilizes the patient’s own T cells to treat cancer. For this treatment, T cells are isolated from the patient, modified to enhance their capabilities of fighting cancer in the lab and reinfused into the patient. At Minerva Imaging we offer a wide range of advanced animal models that mimic human diseases to our clients, and we wish to expand this catalogue with a mouse model for adoptive cell therapy.
In this project, Celine Christine Jensen investigated adoptive cell therapy in preclinical mouse models in combination with standard of care cancer treatments. T cells were isolated from mice, stimulated and expanded in vitro and examined by T cell functional assays. Expanded T cells were subsequently injected into tumor-bearing mice and evaluated for their anti-cancer effect in combination with other cancer treatments. The treatment effect was assessed by tumor growth measurements and analysis of immune cell subsets in tumors using flow cytometry.
Applied methods
- Culturing of cancer cells
- Isolation and culturing of primary T cells
- Functional assays
- Setup of in vivo mouse models by subcutaneous implantation of cancer cells
- Treatment with T cells and relevant cancer therapies
- In vivo tumor growth monitoring by caliper measurements
- Ex vivo evaluation of tumor microenvironment using flow cytometry
Mouse models are an essential tool within the biomedical research area and are used for development of many therapies, including immunotherapies. However, they naturally differ from humans in various ways and there is a need for advanced preclinical models that mimic interactions between human immune responses and tumors and thereby strengthen the evaluation of immunotherapies. Humanized mice can be used to address this issue since these are mice engrafted with human immune cells. Lately, there has been a growing demand for these preclinical models especially within immuno-oncology research. At Minerva Imaging we offer research using humanized mouse models to our collaborators and wish to expand our knowledge and capabilities within this area.
In this project where Louise Marie Jønsson worked as a student assistant, immunodeficient mice were humanized by engraftment of humane stem cells or peripheral blood mononuclear cells (PBMCs) and engrafted with a human tumor. During the course of humanization, the mice were continuously analyzed by flow cytometry for the presence of human immune cells in peripheral blood.
Applied methods
- Setup of in vivo humanized mouse models by engraftment of humane stem cells or PBMCs
- Blood sampling
- Ex vivo engraftment analysis of blood samples using flow cytometry
- Cell culturing of cancer cells
- Subcutaneous implantation of cancer cells
- Treatment with cancer therapies
- In vivo tumor growth monitoring by caliper measurements
- Ex vivo evaluation of tumor microenvironment using flow cytometry and potentially immunohistochemistry
Student: Adele Robdrup Jacobsen is a Master student in Economics and Business administration – Supply Chain Management at Copenhagen Business School (CBS). The project is supervised by Jacob Jacobsen, member of the board of Minerva Imaging and Senior Lecturer Hans-Joachim Schramm from CBS.
Background: The aim of this master thesis is to analyze supply chain management tools and strategies, using Minerva as a case company. The research utilizes a single case study to explore how existing theories for improving operations and supply chain performance can be adapted for use by a fast-growing radiopharmaceutical company, operating under stringent industry regulations and with just-in-time production constraints. The study presents a theoretical framework and analyzes the implications for the case company.
Method: The methodology combines growth theory with traditional supply chain tools and adapts an abductive approach to compensate for data limitations arising from rapid growth of a new business activity. Applied models and analyses include business process mapping, Greiner’s growth model and multi-criteria ABC analysis. Employing a pragmatic research philosophy, the study extensively tests the effectiveness of the selected tools using mixed methods and concurrent triangulation. This approach ensures robust findings despite limited historic data available.
Chronic obstructive pulmonary disease (COPD) refers to a group of progressive lung diseases including chronic bronchitis and emphysema. The underlying molecular mechanisms are poorly understood, and the presentation and progression of disease are varied, though damaged lung tissue, inflammation, and impaired respiration are common denominators. Treatment options today are few, and often only involve relieving symptoms, highlighting the need for further research within the area.
Current pre-clinical COPD models suffer from large variations in lung damage and fibrosis and also suspected “off-target” tissue damage. At Minerva Imaging we would like to conduct a study investigating options of refining the bleomycin induced COPD model and the model induction traditionally performed by dosing bleomycin in the trachea directly or by inhalation of droplets placed at the tracheal opening. The model refinement evaluation will be based on welfare parameters, reproducibility and distribution of lung fibrosis using CT imaging and histology. Furthermore, the model will be used to test a positive control treatment to evaluate longitudinal treatment effect of a compound using CT imaging. Ultimately, tissue for hydroxyproline analysis and histology will be sampled and bronchoalveolar lavage will be performed to look at inflammatory cells using flow cytometry.
Applied methods
- Setup of in vivo model in rodents
- CT imaging
- Flow cytometry
- Image analysis
- Histology and tissue assays
Finalized - Development of an animal model of heart failure with preserved ejection fraction in mice

Heart Failure with preserved Ejection Fraction (HFpEF) is a type of heart failure where the heart muscle contracts normally but is too stiff to relax properly during diastole – leading to impaired filling of the heart and thereby reduced cardiac output. This results in the heart not being able to pump enough blood to meet the body’s needs and causes symptoms such as fatigue, shortness of breath, and edema. HFpEF typically affects elderly people and postmenopausal women, and given the rise in predisposing factors (hypertension, diabetes, and obesity) the global prevalence is increasing. Despite the substantial impact of life quality for patients and great socioeconomic costs, the pathophysiology of HFpEF is poorly understood and there is no curative treatment. Treatment options are limited to management of symptoms and underlying conditions, hence there is an imperative need for ongoing research.
With this project, we seek to develop a mouse model of HFpEF at Minerva Imaging. The aim is to explore and compare two different methods of model induction and investigate disease development through various imaging modalities such as cardiac MRI, PET and SPECT to monitor the progression of the disease. Based on the results we will refine the model to optimize translation to the human variant of the disease.
As a master student you will be part of our internal Minerva Imaging Scholar Program. The program is designed to support Minerva Imaging’s continued development as a scientifically driven CRO and focuses on developing novel tools and procedures that expand the capabilities in our focus areas.
The programs we offer are often in areas where we see value in expanding our services for the benefit of our partners and healthcare overall. So, the work you do will often have an application in important drug development and you will be working with scientists and other co-workers who can guide and inspire you in your work efforts.
Applied methods
- Setup of in vivo mouse model
- Echocardiography
- MRI, PET and SPECT imaging
- Image analysis
- Histology and tissue sampling
Project description
Evaluation of tumors in preclinical models is essential for cancer drug development. Most preclinical research with animal models is performed where tumors are placed subcutaneously. This allows easy implantation and easy evaluation of tumor growth using calipers. However, to better mimic the clinical setting and thus translation to the clinic, tumors may instead be placed orthotopically. Doing so may require complicated surgeries and evaluation by advanced imaging methods. At Minerva Imaging we offer a wide range of advanced animal models that mimic human diseases to our clients, and we wish to expand this catalogue with more orthotopic cancer models.
In this project, subcutaneous and orthotopic cancer models will be compared. The models will be compared based on differences in tumor growth. Additionally, one model will also be evaluated for differences in treatment effectiveness and tumor immune microenvironment composition. To better understand the potential difference in treatment effectiveness, cancer antigen-specific cytotoxic T cells will also be evaluated using flow cytometry.
Applied methods
- Culturing of cancer cells
- Surgical orthotopic inoculation of cancer cells
- Subcutaneous inoculation of cancer cells
- Treatment with anti-cancer therapy
- In vivo tumor growth monitoring by caliper measurements
- In vivo tumor growth monitoring by magnetic resonance imaging (MRI)
- Ex vivo evaluation of tumor microenvironment using flow cytometry
Student
Ida Thorøe Michler is a science student enrolled at the MSc in Human Biology at The University of Copenhagen. She is doing a nine month Master’s thesis project (50 ECTS) at Minerva Imaging. This is a joint project supervised by Sigrid Cold and Carsten Haagen Nielsen at Minerva Imaging and Prof. Andreas Kjær at Cluster for Molecular Imaging (BMI) at UCPH.
Background
The aim of this Master’s project is to generate immortalized cell lines from patient derived xenograft (PDX) models of glioblastoma and breast cancer origin. PDX models are initiated by transplanting tumor pieces obtained from patients directly into mice without the need for in vitro cultivation. While PDX models closely represent the heterogeneity and drug response of patient tumors, they are costly, require extra mice for expansion steps, and take a long time to grow. Replacing these models with PDX derived immortalized cell lines will provide a cost-effective, faster and more translatable tool for conducting pre-clinical studies in vivo and in vitro, while also reducing animal usage in line with 3Rs and good animal welfare practices.
Study description
Cells from subcutaneous PDX tumors will be isolated and cultivated to generate immortal cell lines. Once established, the viability and growth potential of these cell lines will be evaluated after multiple thaw- and freeze cycles to ensure their suitability for long term use. The cell lines will be characterised by using flow cytometry to determine the ratio of human/mouse cells after murine depletion and to quantify relevant target proteins, confirming that the identity of the immortalized cells matches that of the original PDX.
Once characterized, tumor-formation ability will be investigated by inoculating cells either subcutaneously or orthotopically (at the site of origin) in immunodeficient mice and monitoring tumor growth by external calliper measurements or T2*-weighted magnetic resonance imaging (MRI). Similarities between the original PDX models and the PDX-derived cell lines will be investigated with immunohistochemistry. Cell lines will be transfected with the luciferase gene and cell growth and viability after anti-cancer treatments will be measured by bioluminescence. This also allows tumor growth and metastasis to be monitored using bioluminescence instead of the more time-consuming MRI. Finally, qPCR will be used to evaluate methylation status as an indication for cell line resistance to chemotherapy.
Students
Signe Svenning Grønager is a vet student enrolled in the MSc in Veterinary Medicine at The University of Copenhagen. She is doing a nine month long Master’s thesis project (30 ECTS) at Minerva Imaging.
Michala Nordfalk is a science student enrolled in the MSc in Human Biology at The University of Copenhagen. She is doing a nine month long Master’s thesis project (50 ECTS) at Minerva Imaging.
Signe and Michala are both co-supervised by Mette Flethøj Madsen at Minerva Imaging and Prof. Andreas Kjær at The Department of Clinical Physiology, Nuclear Medicine & PET at Rigshospitalet and Cluster for Molecular Imaging at The University of Copenhagen.
Background
Myocardial Infarction (MI), also called heart attack, is when a blockage within the coronary artery reduces or stops blood flow to the heart muscle and causes muscle damage due to lack of oxygen. Acute myocardial infarction affects more than 3 million people globally per year and is a leading cause of death in the Western world (1). Rat MI models are often used for long-term pharmacological studies to investigate recovery processes of the infarcted area. In these rats, MI is currently induced by open thorax surgery with either transient occlusion (30-60 minutes) or permanent ligation of the left anterior descending (LAD) artery (2,3). These models are quite invasive, have high variation in infarction size, and are associated with a high mortality rate of 15-38% (4–6).
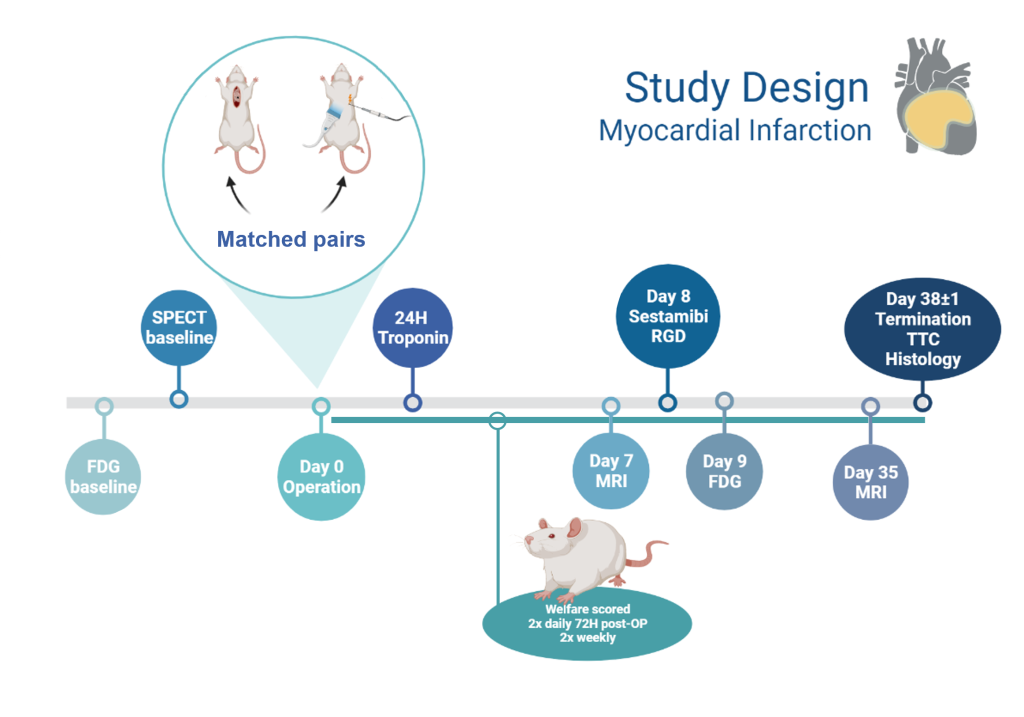
Study Description
Signe and Michala will work together to establish and validate a new minimally invasive MI model in rats using ultrasound-guided electrocoagulation of the LAD artery. This method allows permanent blockage of the coronary artery without open surgery, providing the advantage that intubation and thoracotomy are avoided to improve the overall welfare of the animals. Once the surgical methods are established, each student will continue with separate projects to validate and characterise the new model according to their scientific backgrounds and interests. This collaboration will provide Minerva Imaging with new imaging workflows, lab methods, data analysis, and related protocols for our commercial activities within the cardiac space.
Michala’s Project
The establishment of the minimally invasive model will be compared to permanent LAD ligation one and five weeks after induction using current gold standard MRI methods to measure infarct size and ejection fraction. In addition, angiogenesis and repair within the damaged tissue will be quantified by arginine-glycine-aspartic acid (RDG)-PET/CT and tissue fibrosis will be quantified with late gadolinium enhancement MRI. For further characterization of the model, blood samples will be collected 24 hours post-operation for ELISA quantification of troponin (tissue damage biomarker (7)) and heart samples collected at study end will be stained with the redox indicator, triphenyltetrazolium chloride (TTC) to confirm infarct size ex vivo. The heart samples will then be used for immunohistochemical detection of the angiogenesis-marker, integrin αvβ3.
Signe’s Project
The minimally invasive model will be compared to traditional permanent LAD ligation by evaluation of cardiac perfusion since reduced perfusion within the heart correlates with the infarct size and location in vivo. Cardiac perfusion will be measured with fluorodeoxyglucose (FDG)-PET/CT and Technetium 99m sestamibi (MIBI)-SPECT/CT imaging one week post-MI induction. An animal welfare scoring system specific for this experimental model will be established and compared with infarct size and other outcomes. The animals will be closely monitored to identify and treat any complications as well as monitoring pain levels and responding with appropriate pharmacological and veterinary care.
Figure: Myocardial Infarction project overview, timeline created using BioRender.
References
- Global Awareness of Myocardial Infarction Symptoms in General Population. Korean Circ J. 2021; 51(12): 997–1000.
- Wang et al. Self-Gated Late Gadolinium Enhancement at 7T to Image Rats with Reperfused Acute Myocardial Infarction. Korean J Radiol. 2018; 19: 247–255.
- Martin et al. Preclinical Models of Myocardial Infarction: From Mechanism to Translation. Br J Pharmacol. 2022; 179: 770–791.
- Waller et al Serial Magnetic Resonance Imaging of Microvascular Remodeling in the Infarcted Rat Heart; 2001; 103:1564-1569
- Bentsen et al. Myocardial Perfusion Recovery Induced by an α-Calcitonin Gene-Related Peptide Analogue. Journal of Nuclear Cardiology. 2022; 29: 2090–2099.
- Gao et al. PET Imaging of Angiogenesis after Myocardial Infarction/Reperfusion Using a One-Step Labeled Integrin-Targeted Tracer 18F-AlF-NOTA-PRGD2. Eur J Nucl Med Mol Imaging. 2012; 39: 683–692.
- Frobert et al. Prognostic Value of Troponin I for Infarct Size to Improve Preclinical Myocardial Infarction Small Animal Models. Front Physiol. 2015; 6: 353.
Student
Sarah Wilkens Wulff is a vet student enrolled in the MSc in Veterinary Medicine at the University of Copenhagen. She has successfully completed a 6 month masters thesis project at Minerva Imaging (30 ECTS points). This was a joint project supervised by Philip G. J. Pedersen at Minerva Imaging and Prof. Andreas Kjær at the Department of Clinical Physiology, Nuclear Medicine & PET and Cluster for Molecular Imaging, Department of Biomedical Sciences, Rigshospitalet and University of Copenhagen.
Background
Nonalcoholic Steatohepatitis (NASH) is a lifestyle disease where fat accumulation in the liver causes inflammation, fibrosis and reduced liver function (1). NASH is a serious condition that can progress to more severe liver disease, such as cirrhosis, liver failure, and increased risk of liver cancer. It is a growing health concern worldwide, especially in developed countries where obesity and metabolic disorders are prevalent.
NASH is challenging to diagnose as only patients with advanced fibrosis show clinical symptoms. Today the only reliable diagnostic tool is to obtain a liver biopsy for histopathology which is an invasive and potentially risky procedure (2). To avoid this, extensive work has been done to find and validate diagnostic biomarkers or imaging techniques but still none of these efforts have proven to be sufficiently reliable.
Different Fibroblast activation protein inhibitors (FAPI) are currently being investigated as targets for diagnostic imaging and therapy within both oncology and fibrotic liver diseases (3-5).
Study description
The aim of this Master’s project was to test 68Ga-FAPI PET/CT imaging as a diagnostic tool to quantify liver size, density and liver fibrosis in preclinical rat models. NASH was induced in the rats either by feeding a choline-deficient L-amino acid defined (CDAA) high fat diet (HFD) for 12 weeks, after which time they developed liver steatosis (or abnormal fat accumulation), fibrosis and inflammation (6), or by surgical bile duct ligation, a consistent and repeatable model of liver fibrosis (7).
Results
The result of the study showed that CDAA-HFD fed rats had a significantly higher uptake of 68Ga-FAPI PET/CT compared to controls indicating the presence of liver fibrosis in these animals. Furthermore, CT image analysis showed that initial liver steatosis reduced liver density followed by a gradual increase in liver density as fibrosis developed, similarly to NASH progression in human patients (8). Fibrosis infiltration of the liver was confirmed using histopathology of tumor biopsies (Sirius red area fraction). As in the CDAA model, a significantly higher uptake of 68Ga-FAPI PET/CT was also seen in rats four weeks after bile duct ligation when compared to SHAM operated controls.
Conclusion
We conclude that non-invasive 68Ga-FAPI PET/CT imaging has the potential to precisely visualize and measure liver fibrosis and liver density in pre-clinical models of NASH and therefore has potential as a diagnostic tool in the clinic for the benefit of patients.
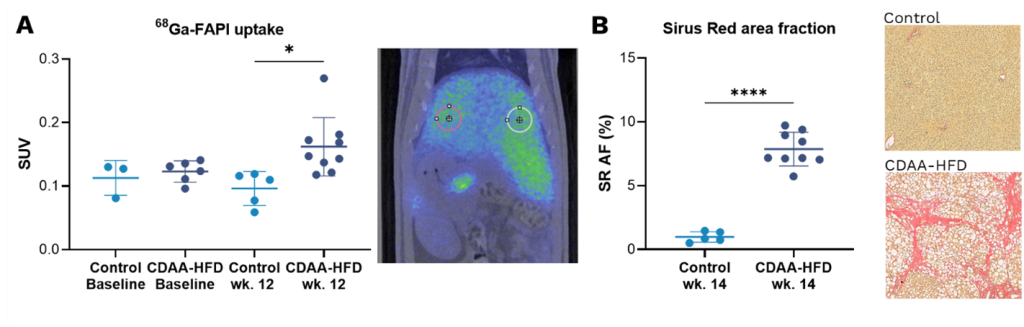
Figure: A) 68Ga-FAPI liver uptake by PET imaging at baseline and after 12 wks. of CDAA-HFD presented as standard uptake value (SUV). B) Sirius red (SR) staining of the liver. Analysed using Visiopharm. All graphs: mean ± SD, n=5-9. ***p<0.001 and ****p<0.0001, by two-tailed t-test.
References
- Hardy et al. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu Rev Pathol. 2016; 11:451-96. DOI: 10.1146/annurev-pathol-012615-044224
- Meyer C et al. Radiation Dosimetry and Biodistribution of 68Ga-FAPI-46 PET Imaging in Cancer Patients. J Nucl Med. 2020 Aug;61(8):1171-1177. doi: 10.2967/jnumed.119.236786.
- Pirasteh A et al. Staging Liver Fibrosis by Fibroblast Activation Protein Inhibitor PET in a Human-Sized Swine Model. J Nucl Med. 2022 Dec;63(12):1956-1961. doi: 10.2967/jnumed.121.263736.
- Wang XL, Li H, Wang QS, Zhang XL. Clinical value of pre-and postoperative 18F-FDG PET/CT in patients undergoing liver transplantation for hepatocellular carcinoma. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Aug;26(8):1087-91, 1095. PMID: 16939890.
- Xiaohan Fang, Man Xie, Youwei Zhao et al. Clinical Value of 18F-FAPI PET/CT in assessing early-stage fibrosis of graft after liver transplantation: preliminary experience, 05 October 2022, PREPRINT (Version 1) available at Research Square [https://doi.org/10.21203/rs.3.rs-2092805/v1]
- Carreres et al. Modeling Diet-Induced NAFLD and NASH in Rats: A Comprehensive Review. Biomedicines. 2021; 9(4): 378. DOI: 10.3390/biomedicines9040378
- Kirkland et al. Reversible surgical model of biliary inflammation and obstructive jaundice in mice. J. Surg. Res. 2010; 164: 221–227. DOI: 10.1016/j.jss.2009.08.010
- Tisch et al. Hounsfield unit values of liver pathologies in unenhanced post-mortem computed tomography. Int J Legal Med. 2019; 133(6): 1861-1867. DOI: 10.1007/s00414-019-02016-1