FAP targeted radiotherapy induces an immunogenic tumor microenvironment
Immunotherapy has proven to be an effective way of treating cancer in the past decade. Furthermore, the role for checkpoint inhibitors like PD-1 has been found of utmost value. More recently, mechanisms of resistance have shown limitations of antibodies targeting PD-1 as monotherapy. Immune-suppressive cells in the tumor microenvironment like the Fibroblast Activation Protein (FAP) play an important role in the resistance mechanism. A recent study by 3B Pharmaceuticals and Clovis Oncology in collaboration with Minerva Imaging, shows that FAP targeted radiotherapy induces an immunogenic tumor microenvironment which enhances the efficacy of PD-1 immune checkpoint inhibition.
In vivo biodistribution of 177Lu-FAP-2287 by SPECT/CT imaging
FAP-2287, a closely related surrogate of FAP-2286 targeting mouse FAP, combines a cyclic FAP-binding peptide linked to tetraazacyclododecane tetraacetic acid (DOTA), allowing radionuclide chelation for imaging or therapeutic applications. The biodistribution of 177Lu-FAP-2287 was evaluated in vivo by SPECT/CT imaging following a single dose of 30 MBq 177Lu-FAP-2287 in MCA205-mFAP tumor bearing mice. FAP-2287 was rapidly taken up in tumors with low off-target accumulation and excretion via renal elimination. High tumor uptake and limited uptake in kidney and liver was observed.
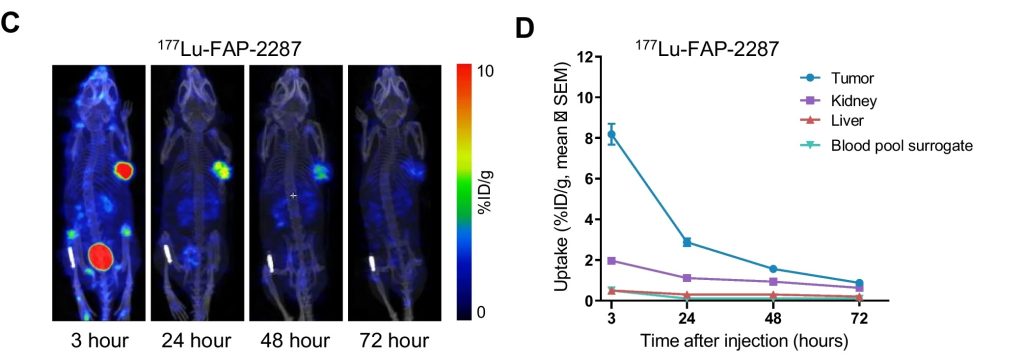
Figure 1: In vivo uptake of 177Lu-FAP-2287 into FAP-positive MCA205-mFAP syngeneic tumors. SPECT images of one representative mouse at 4 different timepoints (C). Quantification of 177Lu-FAP-2287 biodistribution as mean ± SEM %ID/g (n = 6) in tumor, kidney, liver, and blood pool surrogate at 3, 24, 48 and 72 h after injection (D). From Zboralski, D., Osterkamp, F., Christensen, E. et al. Fibroblast activation protein targeted radiotherapy induces an immunogenic tumor microenvironment and enhances the efficacy of PD-1 immune checkpoint inhibition. Eur J Nucl Med Mol Imaging (2023).
High efficacy of 177Lu-FAP-2287 in combination with PD-1
The therapeutic effect of combining anti-PD-1 treatment with 177Lu-FAP-2287 was evaluated in vivo in the MCA205-mFAP tumor model. A significant inhibition of tumor growth was observed for the combined 177Lu-FAP-2287 and anti-PD-1 group with a synergistic effect compared to the monotherapy groups.
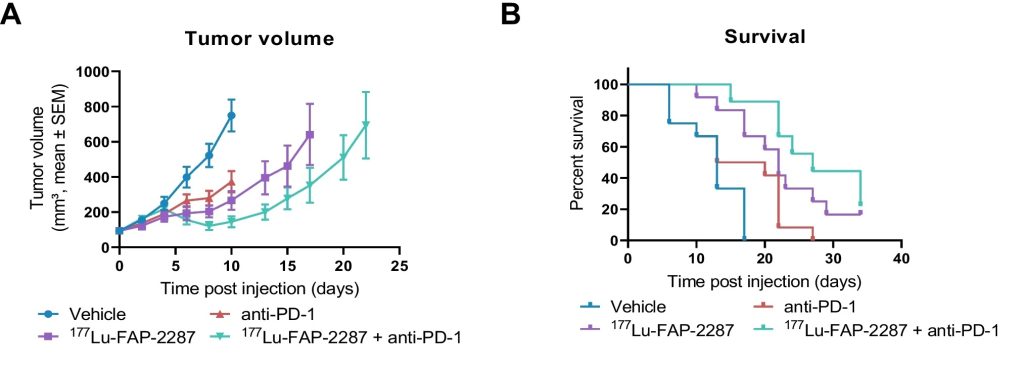
Figure 2: Tumor efficacy of 177Lu-FAP-2287 and in combination with anti-PD-1 in MCA205-mFAP syngeneic tumor model. MCA205-mFAP tumor-bearing mice were treated with vehicle, 177Lu-FAP-2287, anti-PD-1 antibody, or combination 177Lu-FAP-2287 plus anti-PD-1 (n = 12 mice/group). Mean tumor volumes ± SEM during study period (A), Kaplan Meier survival curve (B). From Zboralski, D., Osterkamp, F., Christensen, E. et al. Fibroblast activation protein targeted radiotherapy induces an immunogenic tumor microenvironment and enhances the efficacy of PD-1 immune checkpoint inhibition. Eur J Nucl Med Mol Imaging (2023).
Immune profiling of MCA205-mFAP tumors
The immune infiltration in response to 177Lu-FAP-2287 was investigated by flow cytometry. 177Lu-FAP-2287 increased CD8+ T cell infiltration which was maintained in combination with anti-PD-1 treatment. The increase in CD8+ T cells was accompanied by an induction of STING-mediated type I interferon response and higher levels of co-stimulatory molecules such as CD86.
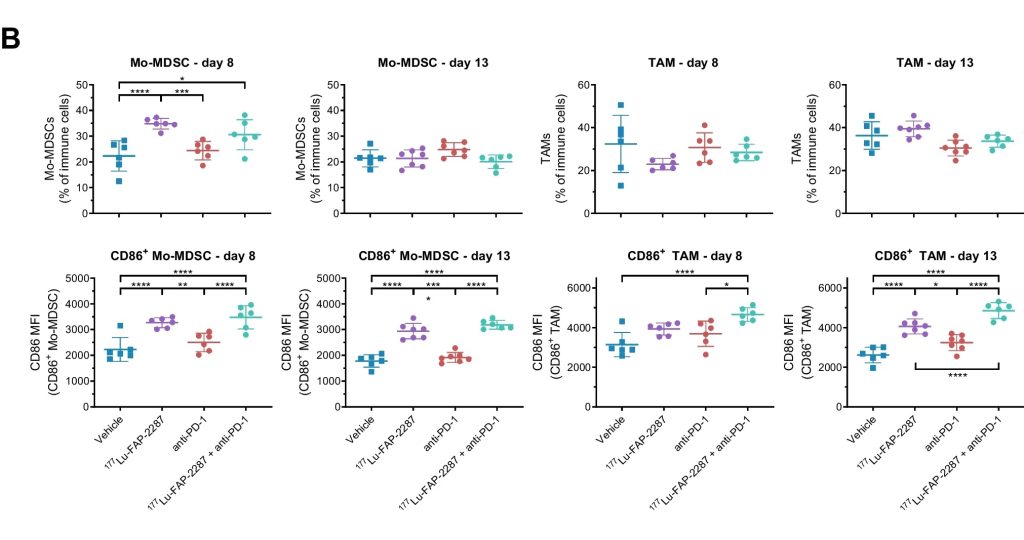
Figure 3: Immune profiling of CD86+ myeloid subsets after treatment with 177Lu-FAP-2287 and in combination with anti-PD-1 in MCA205-mFAP syngeneic tumor model. Individual percentage of Mo-MDSC and TAM and their CD86 MFI with bars as mean ± SEM on day 8 and 13 after treatment. From Zboralski, D., Osterkamp, F., Christensen, E. et al. Fibroblast activation protein targeted radiotherapy induces an immunogenic tumor microenvironment and enhances the efficacy of PD-1 immune checkpoint inhibition. Eur J Nucl Med Mol Imaging (2023).
The study demonstrates that combined therapies using radiolabeled FAP-targeting agents together with PD-1 checkpoint inhibitors, can induce tumor inhibition and prolong survival. The efficacy of this combination is mediated by radiation-induced modulation towards an immunogenic tumor microenvironment. The in vivo biodistribution, efficacy, and pharmacodynamics studies including ex vivo immune profiling by flow cytometry were performed at Minerva Imaging.
We would like to thank all the authors for the work and collaboration.
Full publication:
Zboralski, D., Osterkamp, F., Christensen, E. et al. Fibroblast activation protein targeted radiotherapy induces an immunogenic tumor microenvironment and enhances the efficacy of PD-1 immune checkpoint inhibition. Eur J Nucl Med Mol Imaging (2023). https://doi.org/10.1007/s00259-023-06211-6
Click here to see more publications with contributions from Minerva Imaging.
 Back to news
Back to news