A mesothelin targeted thorium-227 conjugate demonstrates efficacy in preclinical models
In a recent study conducted by Bayer in collaboration with Minerva Imaging, the efficacy and combination potential of a mesothelin targeted thorium-227 conjugate (MSLN-TTC ) was evaluated. The conjugate combines a mesothelin-specific antibody with a chelator, enabling stable complexation of Thorium-227. The potential of the MSLN-TTC was evaluated in vitro and in vivo in preclinical models of drug resistant ovarian and cervical cancers, both cell line derived as well as patient derived xenograft (PDX) models.
The expression of the drug efflux pump P-glycoprotein (p-gp) correlates negatively with overall survival in patients with ovarian cancers. Ovarian cancer models OVCAR-8 and NCI-ADR-RES both show MSLN expression, but p-gp expression was only confirmed in NCI-ADR-RES. The expression of MSLN and p-gp was confirmed in the OvCa PDX model ST206B, originating from a patient with metastatic high-grade papillary serous ovarian carcinoma.
In vitro evaluation mesothelin targeted thorium-227 conjugate
In vitro efficacy of MSLN-TTC was demonstrated in both OVCAR-8 (p-gp negative) and NCI-ADR-RES (p-gp positive) cell lines. Most chemotherapeutics show decreased potency in p-gp-positive NCI-ADR-RES model. However, efficacy of MSLN-TTC is not impacted by p-gp expression and it acts additively with docetaxel and doxorubicin in p-gp-negative models in vitro.
In vivo antitumor efficacy mesothelin targeted thorium-227 conjugate
MSLN-TTC inhibited the growth of OVCAR-8 and NCI-ADR-RES tumors in a manor independent of p-gp expression as shown in Figure 1. MSLN-TTC showed almost equal antitumor activity at 2 x 500 kBq/kg and at 2 x 250 kBq/kg in both models. Treatment was well tolerated without significant animal weight loss.
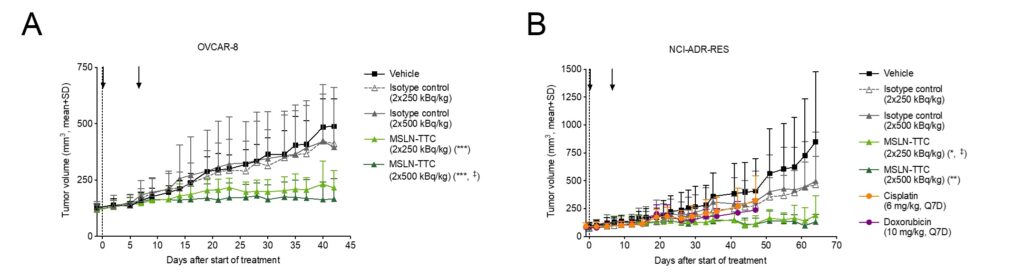
Figure 1: Antitumor efficacy of MSLN-TTC and various chemotherapies. MSLN-TTC was administered as indicated with black arrows. A: Growth curves of p-gp-negative ovarian OVCAR-8 tumors. B: Growth curves of p-gp-positive ovarian NCI-ADR-RES tumors. From Sabine Zitzmann-Kolbe et al, Mol Cancer Ther 2023.
Efficacy in the ovarian cancer PDX model ST206B
MSLN-TTC was further evaluated in combination with docetaxel, pegylated liposomal doxorubicin, regorafenib, and bevacizumab in the ST206B ovarian PDX model. Combination treatments had a stronger antitumor effect compared to monotherapy, as shown in Figure 2 below. All monotherapy and combination treatments with MSLN-TTC were well-tolerated in the ST206B combination study without significant animal weight loss.
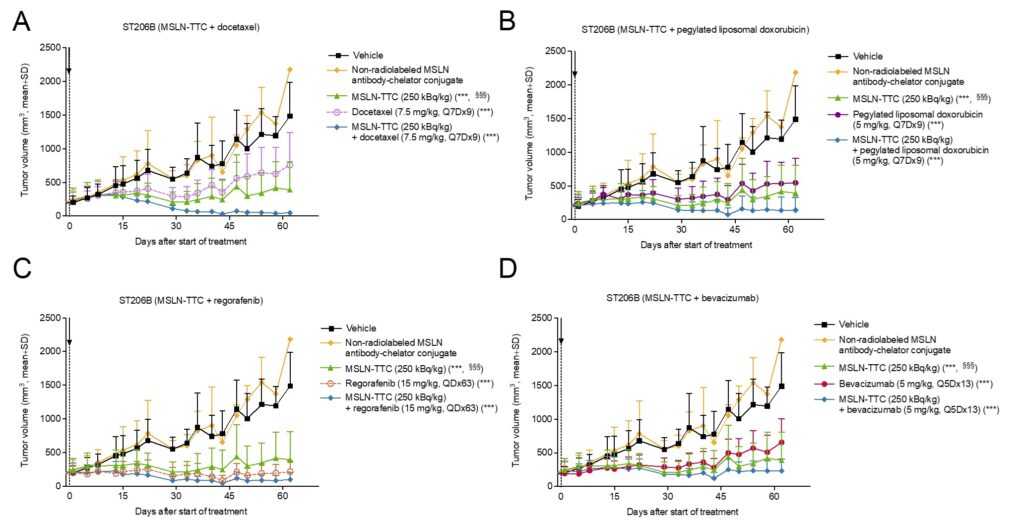
Figure 2: In vivo efficacy of MSLN-TTC as monotherapy and in combination with chemotherapy compounds in the ST206B human OvCa PDX model. From Sabine Zitzmann-Kolbe et al, Mol Cancer Ther 2023.
There is still a high unmet need for patients with high grade ovarian cancers. Standard treatment combines surgery followed by combination chemotherapy with most patients relapsing after therapy. Resistance mechanisms play an important role in the poor therapy effect. P-gp neutralizes the therapeutic effect of several chemotherapeutics, resulting in decreased clinical benefit. In this study, efficacy of MSLN-TTC is shown to be independent of the p-gp status and can thereby overcome mechanisms of resistance to existing therapies for ovarian cancers. Previously, the combination of MSLN-TTC with immune checkpoint inhibitor was reported, demonstrating and immunostimulatory effect of MSLN-TTC as single agent and in combination with anti-PD-L1 therapy (Lejeune P, Cruciani V, Berg-Larsen A, et al).
This publication exemplifies the usage of PDX models in combination with chemo- and anti-angiogenesis therapeutics. Interested to learn how Minerva Imaging can support efficacy studies of targeted radionuclide therapies in relevant PDX models in combination with standard of care therapies and in combination with immune checkpoints inhibitors incorporating flow cytometry as a read out?
Read more about our:
- Flow cytometry services to support all immune monitoring
- Oncology services at Minerva Imaging
- Preclinical targeted radionuclide therapy studies at Minerva Imaging
References:
- Zitzmann-Kolbe S, Kristian A, Zopf D, Kamfenkel C, Politz O, Ellingsen C, Hilbig J, Juul MU, Fonslet J, Nielsen CH, Schatz CA, Bjerke RM, Cuthbertson AS, Mumberg D, Hagemann UB. A targeted thorium-227 conjugate demonstrates efficacy in preclinical models of acquired drug resistance and combination potential with chemotherapeutics and antiangiogenic therapies. Mol Cancer Ther. 2023 Jun 27, https://doi.org/10.1158/1535-7163.mct-22-0808
- Lejeune P, Cruciani V, Berg-Larsen A, et al, Immunostimulatory effects of targeted thorium-227 conjugates as single agent and in combination with anti-PD-L1 therapy. Journal for Immunotherapy of Cancer 2021, https://jitc.bmj.com/content/9/10/e002387.long
 Back to news
Back to news