Minerva Imaging increases its footprint in large animal pharmacology and molecular imaging
At Minerva Imaging we focus on the use of advanced large animal pharmacology models and molecular imaging for translational research and drug development. Molecular imaging includes magnetic resonance imaging (MRI), single photon emission computed tomography (SPECT) and positron emission tomography (PET), and computed tomography (CT). These imaging techniques are widely used in clinical diagnostics and offer the possibility to interrogate biological processes at the cellular level and visualize compound biodistribution in the intact organisms. Over the last two decades we have gained a lot of experience in molecular imaging, and we have on-site capabilities for both small and large animals.
At Minerva we excel in oncology and cardiovascular research and provide services in a wide range of preclinical drug discovery programs. Over the years we established a long list of clinically translational disease models in a variety of small and large animal species. With our current 1500 square meter expansion, Minerva further increases its footprint within large animal pharmacology and molecular imaging.
State of the art facility
The new facility adds more space for housing, a new state of-the-art surgical theater and brings in more imaging capabilities like fluoroscopy and PET-imaging for large animals. With this we aim to increase the model catalogue and imaging endpoints we can offer to our collaborators. In the beginning of 2023, we will open an integrated GMP compliant radiochemistry laboratory, including a cyclotron. As an example, this will enable us to produce 15O-water (very short-lived radioactive version of regular water) for PET-imaging. 15O-water is freely diffusible, and not affected by metabolism and is considered as the gold standard for blood flow imaging and allows absolute quantification. This can be used to interrogate the effect of pharmacological intervention on myocardial blood flow and coronary perfusion in e.g. pig models of myocardial infarction or coronary microvascular dysfunction.
Large animal pharmacology and biodistribution study
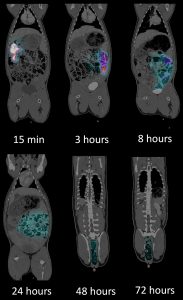
Our in-house radiochemistry facility can label test-particles with the diagnostic SPECT radioisotope like In-111. This enables us to track the in vivo distribution of compounds and obtain comprehensive knowledge of uptake, biodistribution, excretion time and route. In one study we conducted, we delivered radiolabeled particles into the duodenum of Göttingen Minipigs using endoscopic guidance. Quantitative SPECT/CT-imaging was performed 15-minutes, 3-, 8-, 24-, 48-, and 72-hours post duodenal delivery. The images showed that the radioactive labeled particle stayed in the gastrointestinal tract, did not enter systemic circulation, and was excreted exclusively in feces. These results were based on the image analysis and a quantitative output of the wash-out calculated in percent of the injected dose (%ID) over time.
Academic collaborations
As a scientifically driven CRO, our competences build on more than two decades of research within oncology and molecular imaging performed at the University of Copenhagen and Rigshospitalet, the National University Hospital of Denmark. We have collaborators from all over the world, from small biotech to large pharma and our door is always open for academic collaborators. Are you working in academic research and would like to learn more about our capabilities? Please reach out, we are looking forward discussing how we can set up tailor-made collaborations. Contact us for more information.
More information
 Back to news
Back to news